How To Calculate Hdi Chemistry
Degrees of Unsaturation (Index Of Hydrogen Deficiency): How the molecular formula of a chemical compound can give helpful hints about its construction.
Today nosotros'll talk about a quick and useful calculation we tin can perform to obtain the number of [multiple bonds + rings] in an unknown structure merely past knowing the molecular formula. We call information technology the Index of Hydrogen Deficiency, or more than commonly, only "Degrees of Unsaturation".
Here'southward the short, condensed version:
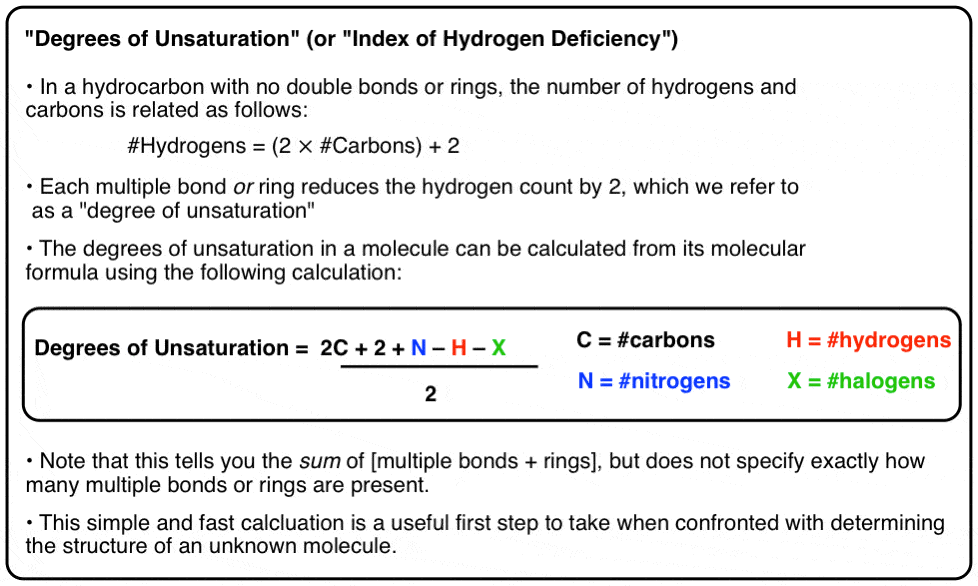
Table of Contents
- What'southward And so Great About The IHD?
- Playing Around With Molecular Formulae: Degrees of Unsaturation
- For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons, Plus 2
- Each Double Bond or Ring Reduces The Hydrogen Count By 2
- Each Ring Or Double Bail Is Chosen A "Caste Of Unsaturation"
- Instance: Benzene (iv Degrees Of Unsaturation)
- What About Molecules Containing Oxygen?
- What About Halogens?
- Nitrogen Is A Lilliputian Fleck Tricky
- A Unified Formula For Degrees of Unsaturation
- Applying The IHD Formula To Four Real-Life Examples
- Quiz Yourself!
1. What'due south So Dandy About The IHD?
The best thing almost the IHD is that it is an piece of cake equation to employ that gives yous useful data nigh the structure of an unknown compound. Information technology literally takes a minute to do.Calculating the degrees of unsaturation is THE first task I recommend doing when y'all are faced with determining the construction of an unknown compound with a known molecular formula.In the final postal service, we saw that this calculation was clutch in the determination of the structure of the side chain of deer tarsal pheromone.
Before we get going, see if you lot tin employ the equation in the box above to notice the degrees of unsaturation in theseextremely well known molecules whose formulae are given below.
- C9H8Ofour
- C21 H thirty O 2
- C 17 H 21 Due north O 4
- C 11 H 15 N O 2
The latter 3 molecules are somewhat "naughty" examples, being illegal in near states. Hopefully that'south an incentive for y'all to really do the calculation! (answers at the bottom of the post).
ii. Playing Around With Molecular Formulae: Degrees of Unsaturation
Let's kickoff this discussion past looking for patterns in molecular formulae. We'll commencement with hydrocarbons containing no double bonds or rings.
Can you lot see a human relationship between the number of carbons and the number of hydrogens?
More than specifically, tin can yous come up upward with a formula?
iii. For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons, Plus ii
You should exist able to meet that for a hydrocarbon with no rings or double bonds, the number of hydrogens is equal to twice the number of carbons, plus ii.
So for a molecule like dodecane (C12) nosotros'd look to see (12 x 2) + 2 = 26 hydrogens.
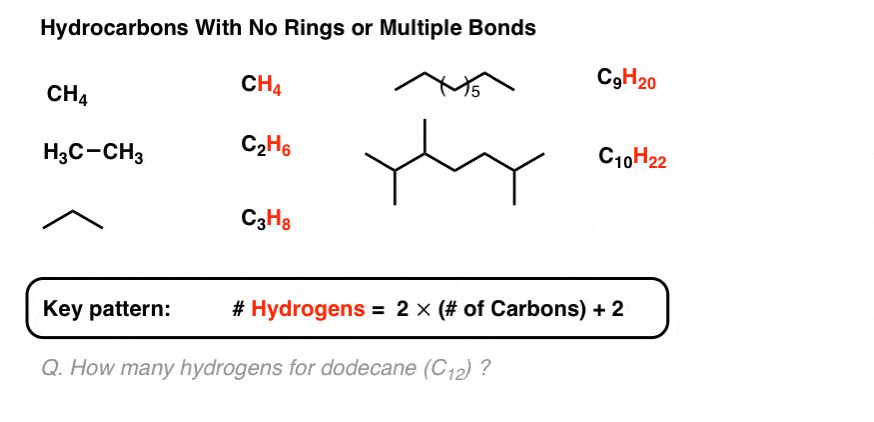
Now: what happens to the molecular formula when we add a double bond to the molecule?
4. Each Double Bond or Ring Reduces The Hydrogen Count By 2
You should exist able to run across that each multiple bond (π bail ) the number of hydrogens in the formula decreases by ii. Ethyne (2 π bonds) has two fewer hydrogens than ethene (1 π bond), which has two fewer hydrogens than ethane (zero π bonds).
Hydrocarbons containing π bonds are often called "unsaturated" hydrocarbons. They can exist treated with hydrogen (Pd/C, Hii ) to give the corresponding alkane with no π bonds, which is then said to be "saturated" with hydrogen. (Compare "unsaturated fats", which contain alkenes, and "saturated fats" which exercise not).
Since every pi bond results in a loss of ii hydrogens from the molecular formula, we refer to this to as a "degree of unsaturation".
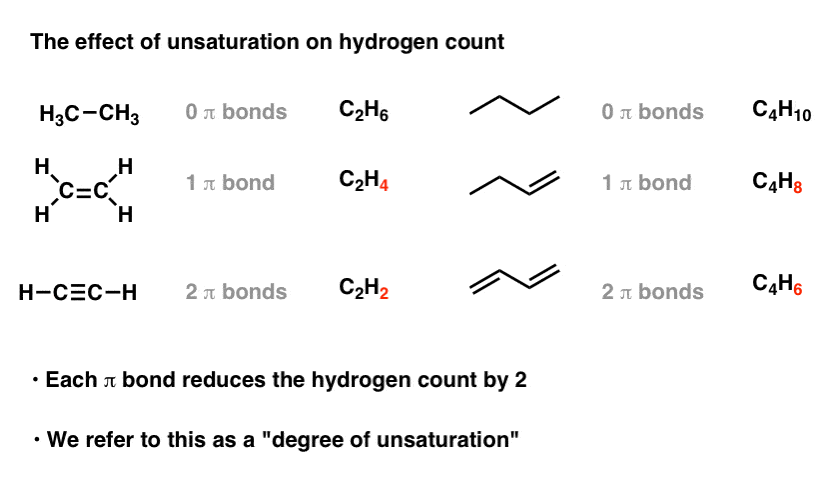
Allow'due south plough our attending to cyclic compounds. Exercise you discover a similar effect?
You should! Every band in the molecule decreases the number of hydrogens by two.
5. Each Band Or Double Bond Is Chosen A "Caste Of Unsaturation"
Therefore, each band introduces a "degree of unsaturation" into the molecule.
Y'all might inquire: what if we have a molecule with rings and multiple bonds? See for yourself.
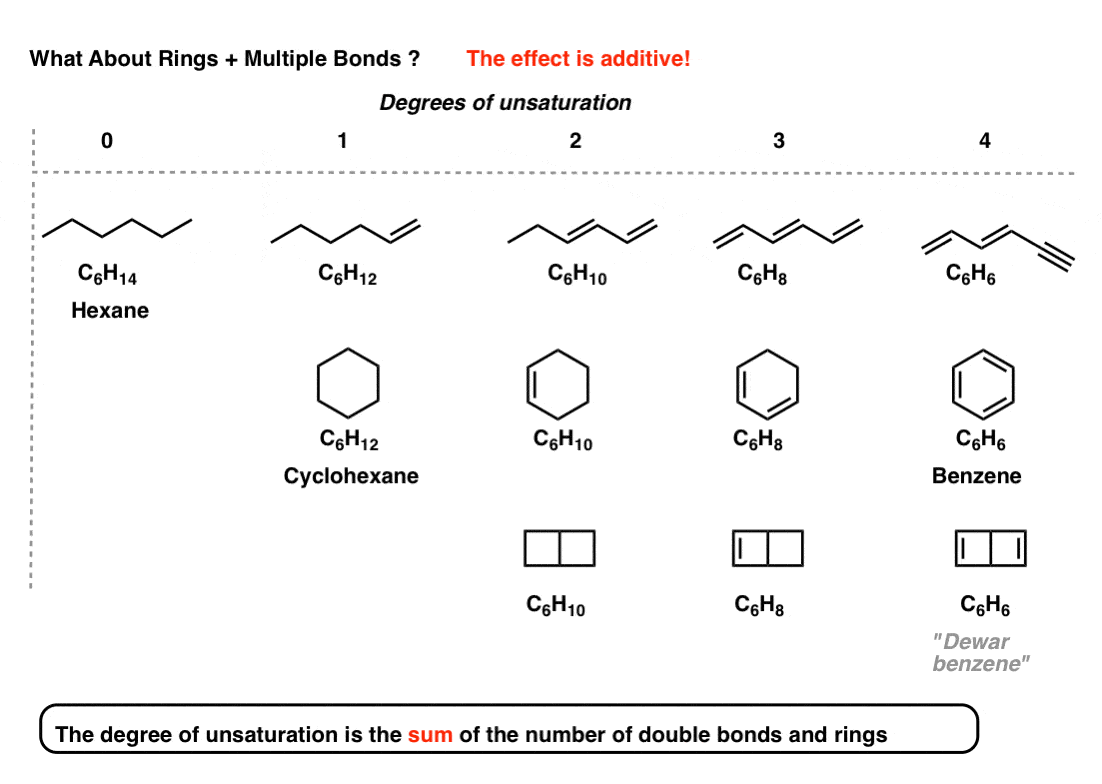
The effect is additive. That is, the "degrees of unsaturation" is thesum of the number of double bonds and rings.
Annotation that it doesn't tell you how many double bonds are present or how many rings are present. It merely tells you their sum.
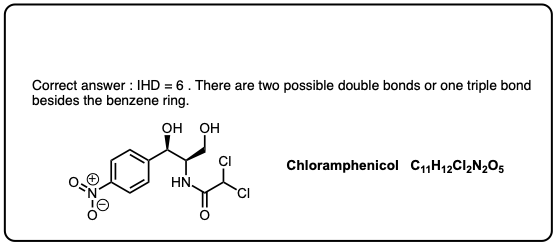
half dozen. Case: Benzene (4 Degrees Of Unsaturation)
For instance, thus the molecular formula C6Hhalf dozen (4 degrees of unsaturation) is satisfied by molecules with
- 4 pi bonds
- iii pi bonds and a ring (benzene)
- two pi bonds and two rings (the very unstable Dewar Benzene, synthesized in 1963)
- ane pi bail and 3 rings (the even more unstable benzvalene, a contact explosive, synthesized in 1971)
- and yep, even zero pi bonds and 4 rings (prismane, synthesized in 1973).
[Historical side notation: the formula of benzene was known to exist C6H6 from Michael Faraday in 1825, merely the correct structure was non proposed until 1865 (Kekule) and non confirmed until 1929, by Catherine Lonsdale's X-ray studies [h/t BRSM]. Just a reminder that knowing the molecular formula but gets you so far. ]
Helpful hint: if you see four degrees of unsaturation in an unknown molecule, thinking "benzene ring" is a good place to start.
7. What About Molecules Containing Oxygen?
And then far, so skillful. Let's move on. What well-nigh molecules with oxygen?
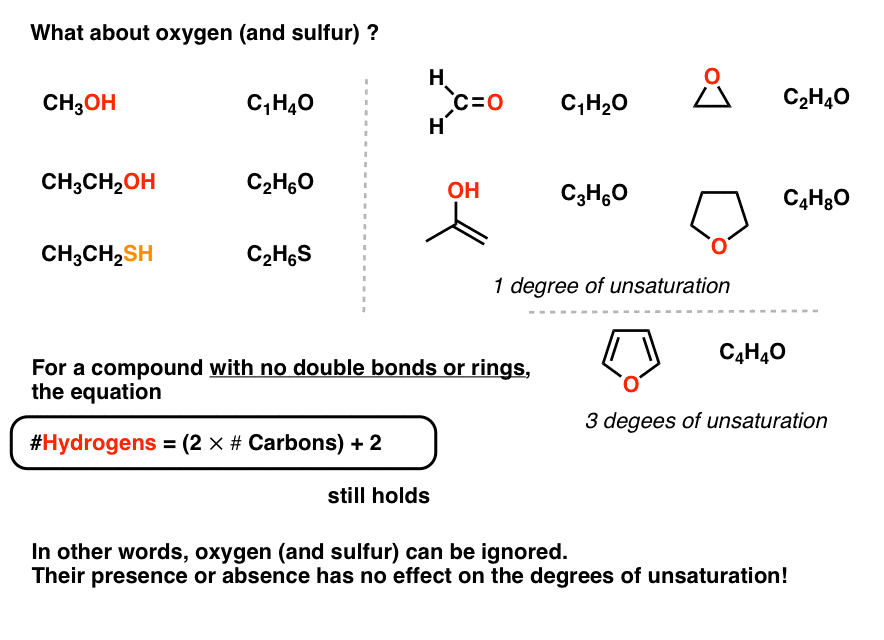
The aforementioned rules apply! We can summate the number of hydrogens from the number of carbons as if the oxygen didn't be.
Annotation that a π bond which contains oxygen still counts as a "degree of unsaturation" (come across formaldehyde, CH2O).
8. What About Halogens?
OK. What about halogens similar chlorine, fluorine, iodine and bromine?
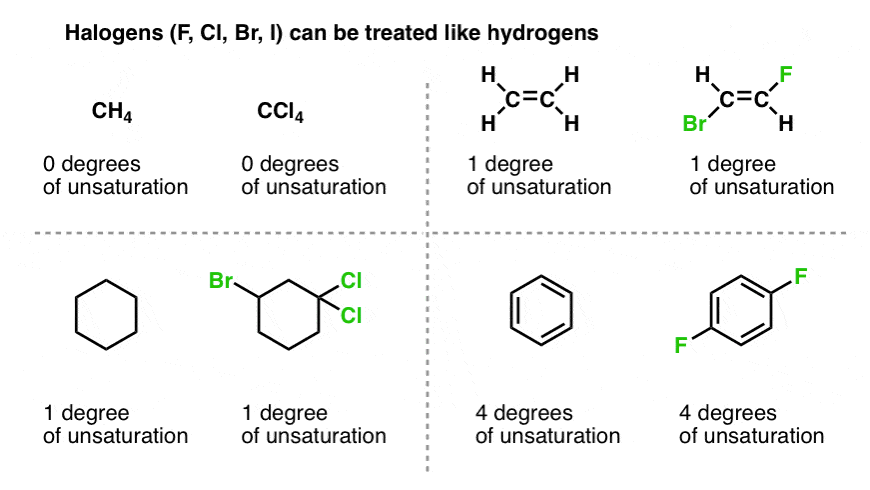
For the purposes of our formula relating hydrogens to carbons for molecules without rings or double bonds,halogens can be counted every bit hydrogens.That is, carbon tetrachloride (CClfour) has the same degrees of unsaturation (zippo) every bit CH4.
We can thus alter the equation for a molecule with no double bonds or rings as follows:
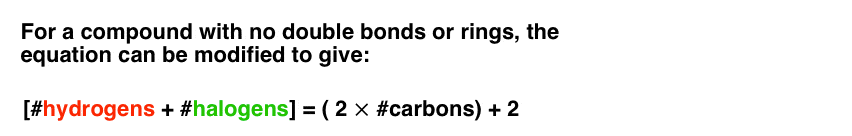
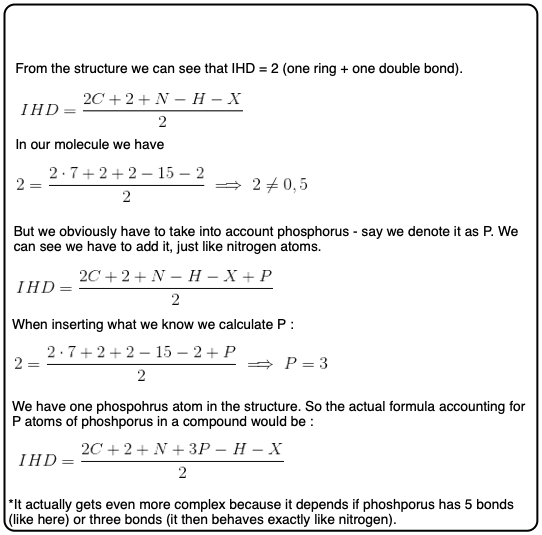
nine. Nitrogen Is A Picayune Flake Catchy
We've saved nitrogen for last, because it's a bit weird. Try to see the pattern of how nitrogen affects our formula.
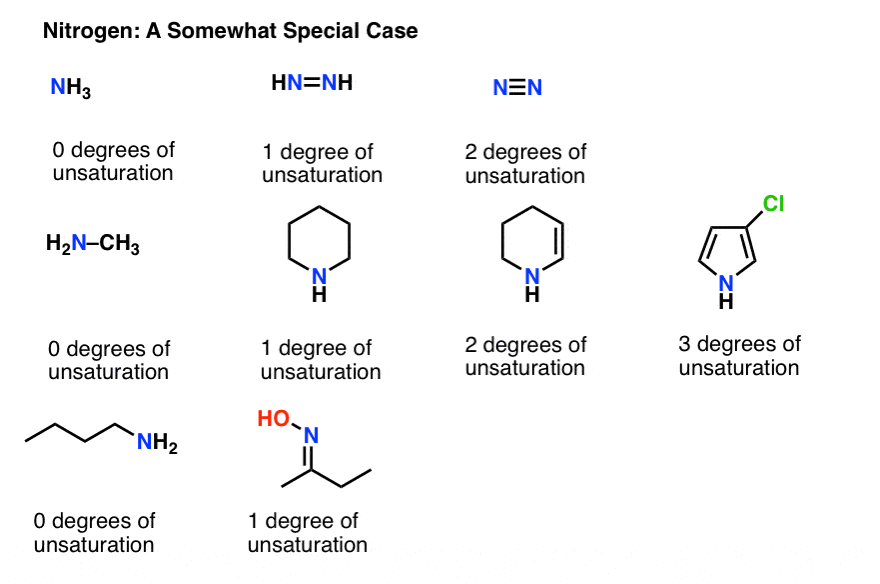
OK, y'all might take noticed that we can't ignore nitrogen like we did oxygen.
The fashion to make the equation work with nitrogen is to add the nitrogen count to the right hand side of the equation, like this:
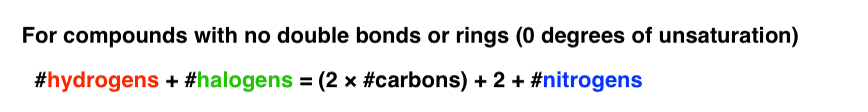
Try information technology with cadaverine (CvH14N2) which contains no double bonds or rings.
ten. A Unified Formula For Degrees of Unsaturation
And so far our equation has only applied to "saturated" compounds with no double bonds or rings.
How can we utilize this to make a general formula for "degrees of unsaturation", which is actually… useful?
Math time. If we movement the left hand side over to the right, we go this:
![]()
Zero equals the degrees of unsaturation in this instance.
If nosotros introduce a double bond or ring, the equation volition return "2".

Each successive caste of unsaturation will increase this number past 2. For example, 2-chloropyridine C5H4NCl gives united states of america viii:

At present 8 is not the degrees of unsaturation. It'due south merely the number of hydrogens "missing" from the respective non-circadian alkane of the same carbon count.
In order to make this equation give united states of america the bodily degrees of unsaturation, we need to divide everything past ii.
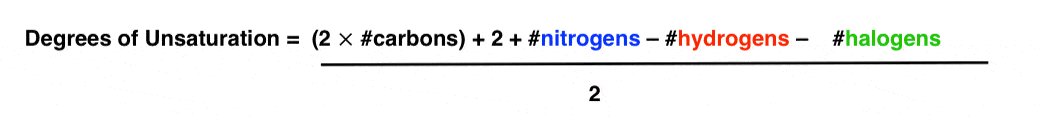
We can really condense things by using the symbols H, C, N, and X to correspond hydrogens, carbons, nitrogens and halogens, respectively.
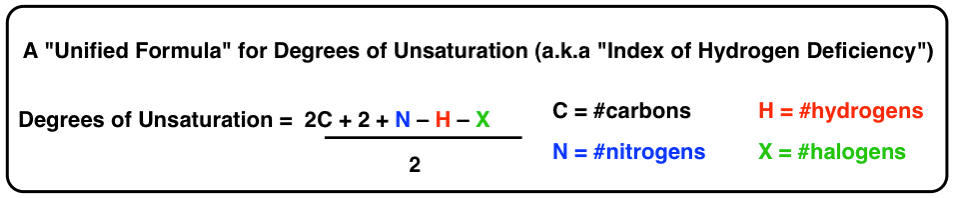
This is our final form for the equation for "degrees of unsaturation", or "index of hydrogen deficiency" if yous prefer.
11. Applying The IHD Formula To Iv Real-Life Examples
OK, now for those existent-life examples I mentioned in a higher place.
- CixH8O4
- C21 H thirty O 2
- C 17 H 21 Northward O4
- C eleven H 15 Due north O ii
Allow'due south plug each of them into our formula. You should get:
- CixH8O4[vi degrees of unsaturation]
- C21 H 30 O two[7 degrees of unsaturation]
- C 17 H 21 North O4 [8 degrees of unsaturation]
- C 11 H 15 N O 2[5 degrees of unsaturation]
Now, let's wait at each of the molecules. You'll observe that the calculated degrees of unsaturation agrees with the sum of [multiple bonds and rings] in the molecule.
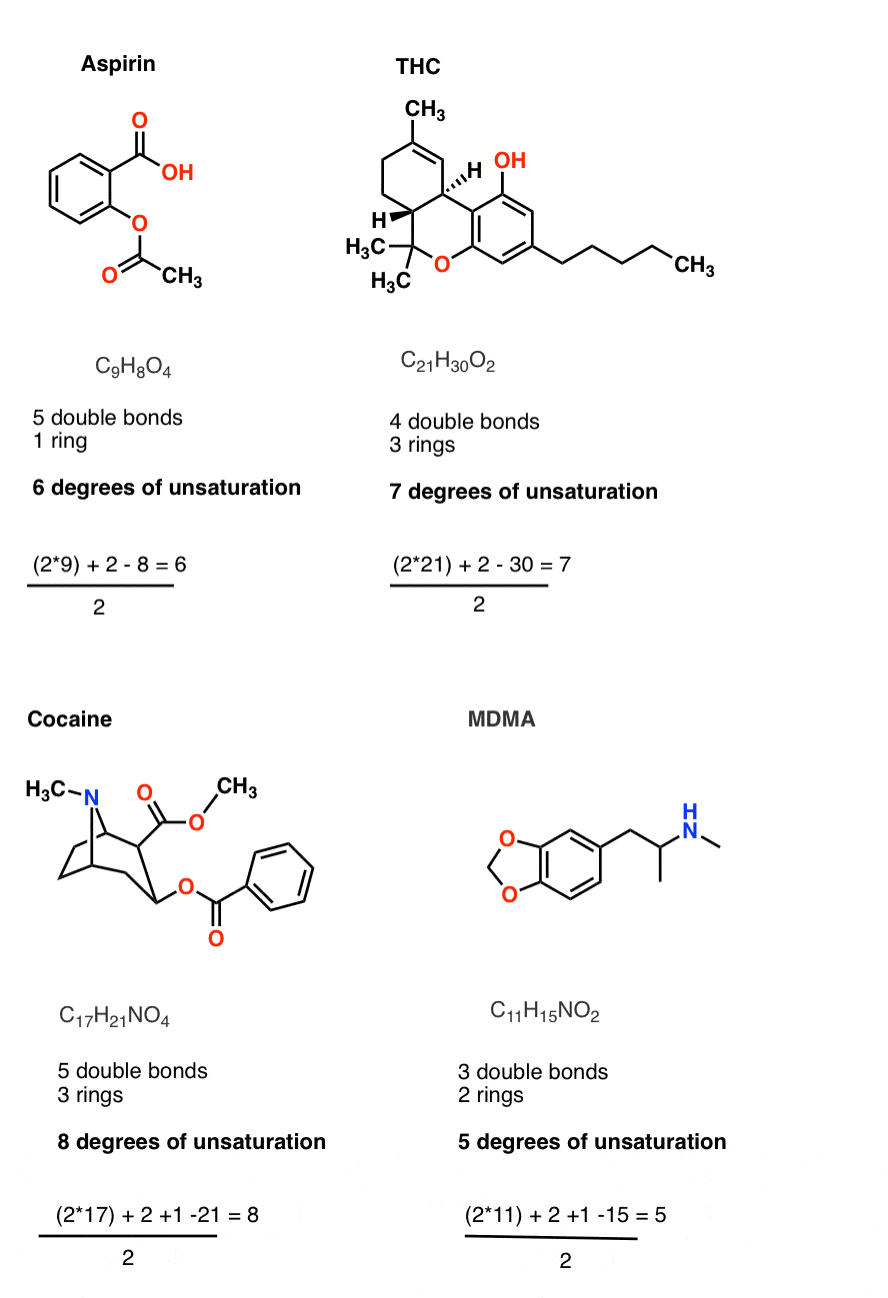
Super useful. Super like shooting fish in a barrel.
With the structure in front of you it seems obvious. Simply if you're dealing with an unknown information technology can be very helpful, similar nosotros saw with deer tarsal gland hormone in the last post.
In the adjacent post, we'll start delving into the mysteries of spectroscopy. We'll start with one of the simplest spectroscopic techniques – UV-Vis spectroscopy – and show how it can be used to deliver clues about the structures of diverse molecules.
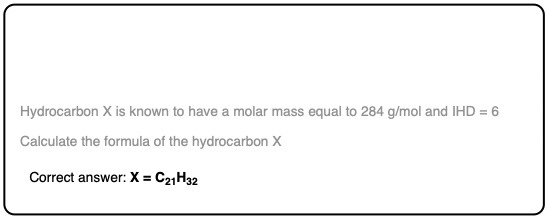
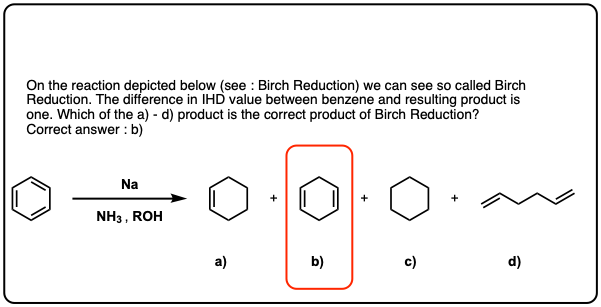
Quiz Yourself!
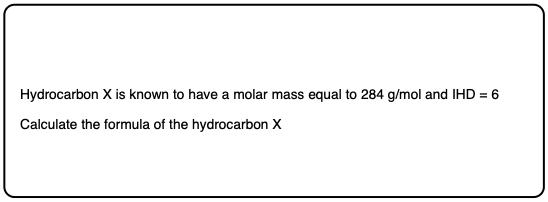 Click to Flip
Click to Flip
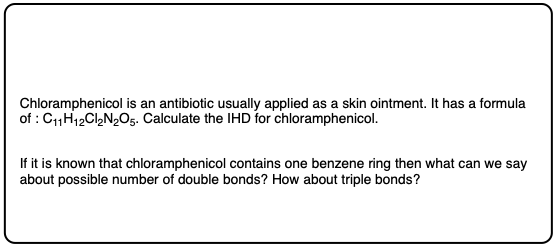 Click to Flip
Click to Flip
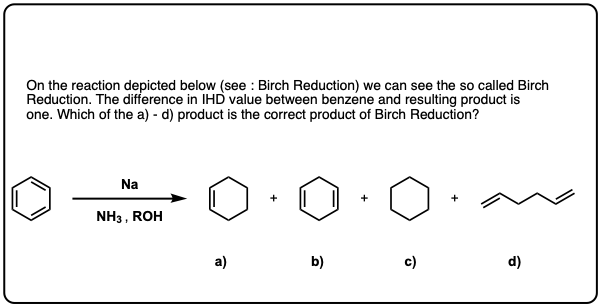 Click to Flip
Click to Flip
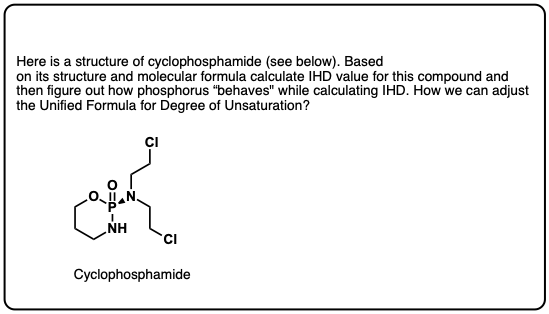 Click to Flip
Click to Flip
How To Calculate Hdi Chemistry,
Source: https://www.masterorganicchemistry.com/2016/08/26/degrees-of-unsaturation-index-of-hydrogen-deficiency/
Posted by: leeyoutive.blogspot.com


0 Response to "How To Calculate Hdi Chemistry"
Post a Comment